Early-stage medical device company, Cardio Flow, Inc. based out of St. Paul, Minnesota received $4.3 million in equity funding from an undisclosed angel investor in early June. This funding coincides with the company’s engagement in their First-in-Human (FIH) clinical trial.
Founded in 2014, Cardio Flow, Inc. is run by CEO, Michael J. Kallok, Ph.D., a University of Minnesota trained biomedical engineering and veteran in the medical device industry, along with a Board of Directors staffed by a range of experts in medical technology development and marketing. Cardio Flow’s team of medical device engineers are improving and expanding upon the revolutionary work started decades prior in orbital atherectomy technology by the late cardiologist and inventor Dr. Leonid Shturman. Cardio Flow currently owns Dr. Shturman’s vast intellectual property portfolio that includes 27 issued U.S. patents as well as IP protections in Europe, Canada, and Australia.
Atherosclerosis is a chronic, inflammatory condition characterized by the progressive build-up of deposits of cholesterol, fatty substances, cellular debris, minerals and clotting components in the blood on the inner walls of arteries. This thickening layer of plaque narrows the channel diameter of the vessel, reducing blood flow thus the levels of oxygen and nutrients transported in blood from reaching the body’s organs and tissues. This build-up could lead to coronary, peripheral and/or carotid artery diseases which are major risk factors for strokes, heart attacks, organ failure and gangrene-associated loss of limbs.
Atherectomy systems are designed to remove this build-up in vessels and restore adequate circulation. However, devices currently on the market have proven ineffective at removing the different forms of plaque deposited, particularly in the larger arteries of the neck, torso, and legs.
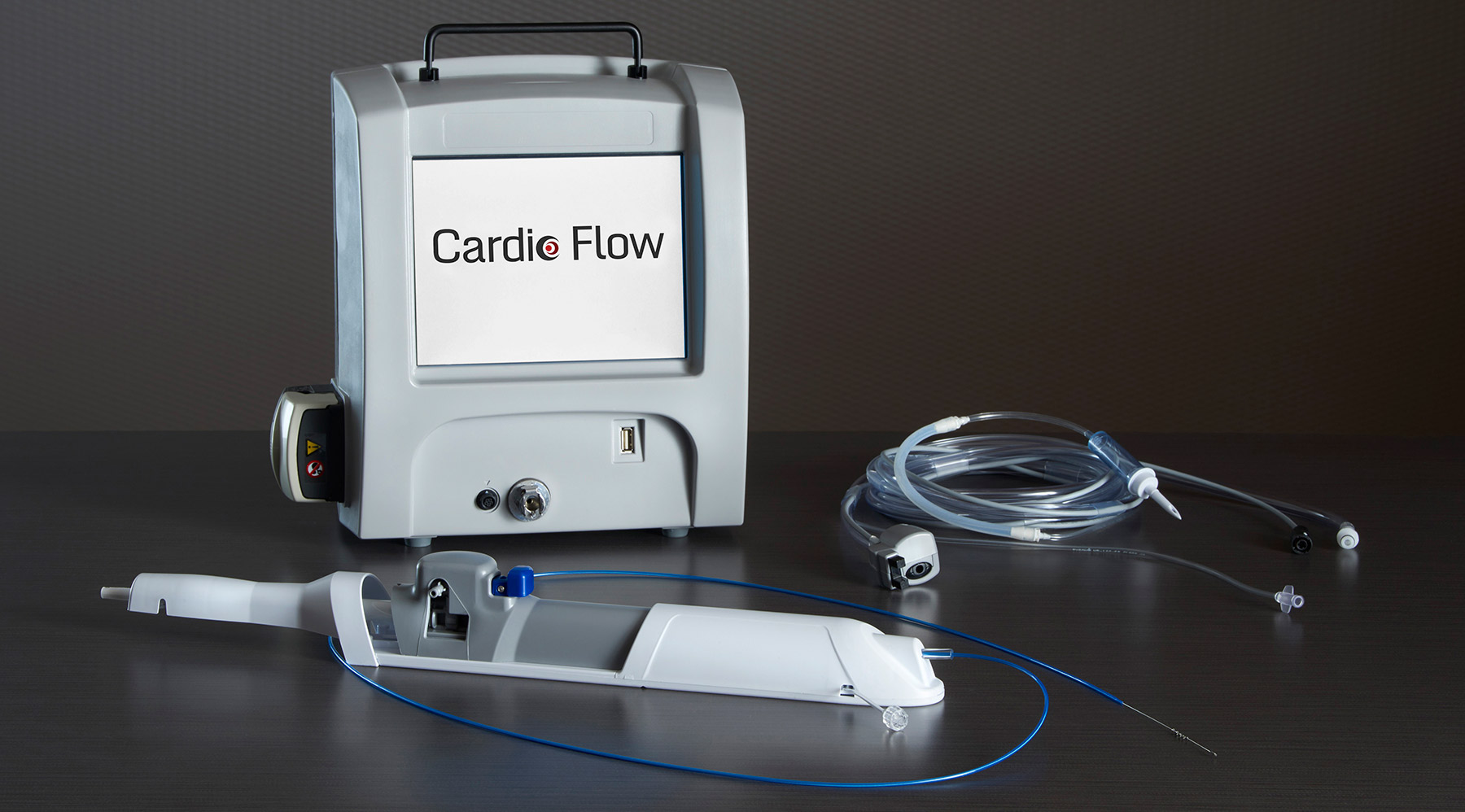
Cardio Flow’s FreedomFlow™ Orbital Atherectomy device is the first to provide physicians with a potentially thorough means of removing not only both types of plaque – the more resistant calcified plaque as well as the softer fibrotic plaque – that can buildup on the walls of blood vessels leading to blockage and poor blood flow, but of also doing so in vessels of a wide range of dimensions, from coronary and peripheral arteries to even the more difficult-to-treat large-diameter carotid arteries.
According to the company’s website, their atherectomy device consists of a driveshaft to which multiple spheres, coated with 50 micron-sized diamond dust, are “eccentrically” attached providing greater surface area for interaction, which when rotated in “orbital technique” adjusts to the size of the blood vessel and more efficiently removes the plaque build-up. Orbital speed, as well as peristaltic pump and saline flush activity, are all regulated via a portable touchscreen control module.
The current feasibility clinical trial is on the removal of atherosclerotic plaque in peripheral vessels of the lower extremities. This trial will assess the safety and efficacy of the Cardio Flow orbital circumferential atherectomy device and inform future development strategies for the FreedomFlow™ system.
For additional information about this company, visit www.cardioflow.net